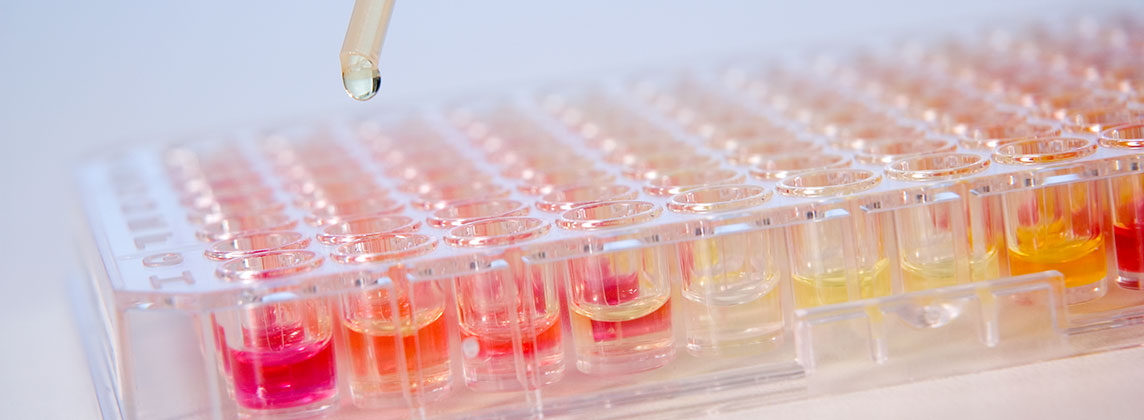
MICRONAUT antimicrobial susceptibility testing (AST)
The test principle of the MICRONAUT system for antimicrobial susceptibility testing (AST) is based on phenotypic detection of resistance as expressed by microbial growth in presence of antibiotic compounds. This micro dilution procedure is a standardized method and a worldwide accepted reference system for determination of the minimal inhibitory concentration (MIC).
For all MICRONAUT AST systems the various antibiotics are placed with or without broth in a dehydrated form into the wells of the microtitration plates and are dissolved by adding bacterial suspensions. After 6 hours (rapid AST) or 18-24 hours of incubation at 35-37°C the AST plate is measured in the photometer and the test is evaluated with the MICRONAUT software or read visually.
Choose your preferred standard like EUCAST or CLSI and compose your own customised antibiogram from more than 200 antibiotics. Otherwise you can select from a wide variety of standard layouts. Take your choice, whether it's breakpoint procedure, MIC testing or a combination of both.
PRODUCTS
MICRONAUT-S
Microplates for automated or manual susceptibility testing of bacteria

Principle of the test
The susceptibility testing with MICRONAUT-S plates is based on the rehydration of antibiotics by adding a standardized bacteria suspension. After incubation of 18-24 hours at 35-37°C the result is read photometrically with the Skan device and evaluated with the MICRONAUT Software or read visually and interpreted.
Storage conditions
Because of a special vacuum drying process the plates can be stored at usual room temperature of 15-25°C. The MICRONAUT-S test plates have a shelf life of 24 months.
Test procedure
- Preparation of bacteria suspension in NaCl (McFarland 0.5)
- Transfer to broth (i.e. Mueller-Hinton II-broth, H-broth)
- Inoculation of MICRONAUT-S test plate
- Incubation for 18-24 hours at 35-37°C
- Photometric reading with the Skan device
- Interpretation of the results with the MICRONAUT software
Antibiotics
Any customer defined antibiotic configuration (MIC or break point) can be offered. Depending on the antibiotics and concentration ranges selected (breakpoint or MIC) 1, 2 or 4 isolates may be tested on a single MICRONAUT-S plate. In addition, customers have the choice to select between a diversity of standard AST panels.
Further details here: Product description MICRONAUT-S
UMIC® product line
High accuracy single drug MIC testing

The UMIC® portfolio comprises six antibiotics and antibiotic-combinations:
- Cefiderocol
- Colistin
- Piperacillin-tazobactam
- Vancomycin/teicoplanin
- Daptomycin
- Linezolid
For more information, please, take a look to the UMIC® product brochure , the UMIC® Cefiderocol flyer or visit Bruker's website
MICRONAUT-AM
MIC plates for the automated or manual susceptibility testing of yeast

The susceptibility testing is based on the rehydration of antimycotics by adding a standardized yeast suspension. After incubation of 24-48 hours at 35-37ºC the plates are ready to be measured photometrically or be interpreted visually. Because of a special vacuum drying process the plates can be stored at a room temperature of 15-25°C. MICRONAUT test plates have a shelf life of 24 months at date of production.
Features of the MICRONAUT-AM system
- Susceptibility testing of up to 9 antimycotics in up to 11 concentrations following the MIC procedure (minimum inhibitory concentration)
- Contains the antimycotics Anidulafungin, Caspofungin and Posaconazol
- The included negative control simplifies the visual as well as the photometrical reading
- RPMI medium improves the growth of the yeast
- Interpretation after 24 hours for yeast and 48 hours for cryptococci
- Interpretation according to EUCAST Guidelines
Procedure
- Preparation of yeast suspension in NaCl 0.9 % (McFarland 0.5)
- Transfer to RPMI medium plus AST indicator
- Inoculation of MICRONAUT-AM test plate
- Incubation at 35-37°C for 24-48 hours
- Incubation period depending on color change (pink) of the growth control
- Photometrical or visual reading of the test plates
Further details provides the product description about MICRONAUT-AM
MICRONAUT-UR
System for bacteriological diagnostics in urinary tract infection (UTI)
NEW PRODUCT DESIGNED FOR BACTERIOLOGICAL DIAGNOSTICS IN URINARY TRACT INFECTIONS (UTI)
Maximum flexibility and reliability for all requirements of the laboratory routine combined in a single system
NEW >> Improved spectrum of antibiotics by introduction of meropenem and the novel antibiotic mecillinam
- Colorimetric identification of 50 taxa of the relevant gram-negative bacilli and grampositive cocci with 23 reactions
- Antimicrobial susceptibility testing of all relevant gram-negative bacilli and grampositive cocci with 20 antibiotics (incl. MRSA detection and ESBL screening)
- Identification and susceptibility testing of 2 isolates combined in one work step based on ideal workflow
- Computer-assisted interpretation after visual or automatic reading
- MICRONAUT software for automated reading, evaluation, interpretation and reports:
- Expert system for verification of MIC data in terms of plausibility and efficacy
- Statistic-module for the statistic evaluation of the generated data
- QC-module for the internal quality control
- Interface for AIS systems in GDT and in LDT format included
- Easy storage (15-25°C) and high product stability (shelf life of 24 month from date of production).
Order directly online
Our sales partner AUROSAN in Essen / Germany is your first contact for these MICRONAUT test products for use in urological diagnostics. You can order the MICRONAUT-UR plates as well as the associated media in their online shop. The AUROSAN team will also be glad to advise you on whether this MICRONAUT system can be used efficiently both diagnostically and economically. Simply contact them directly >> micronaut@aurosan.de
MICRONAUT AST-SYSTEMS
Get here your deeper insight into the testing procedure
Download
Product descriptions